How Does Dalton's Theory Explain the Conservation of Mass
Atoms of different elements differ in mass. Law of Conservation of Mass.

Dalton S Atomic Theory Reading Comprehension Practice Science Teacher Resources Reading Passages
Complete step by step solution-Dalton proposed certain postulates about atomic theory-Matter consists of indivisible atoms-All atoms of a given element have identical properties except identical mass.

. Law off conservation Off mess and the law. All matter is composed of extremely small particles called atoms. 2 Atoms of same element are identical and atoms of different elements are non identical.
Five statements that some of John Daltons theory. In other words mass is conserved in a chemical reaction. In a given compound the relative numbers and kinds of atoms are constant.
Daltons theory was built on this law. While all atoms of an element were identical different elements had at oms of different sizes and masses. S atomic theory explains that the matter is made up of indestructible atoms and that the atoms of one element cannot be destroyed or changed into atoms of another element.
It is clear that when a chemical reaction take place only the atoms present as the reactant are involved in the formation off product. The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy the mass of the system must remain constant over time as systems mass cannot change. Daltons atomic theory helped to explain the law of conservation of mass because it stated that atoms.
But it never gives any details of Law of radioactivity. Daltons theory explains Law of conservation of mass Law of constant composition Law of multiple proportions. Lets through problems in in this problem.
All matter consists of indivisible particles called atoms. The law of conservation of mass law of definite proportion and law of multiple proportions has to be explained on basis of Daltons atomic theory. Chemistry 22062019 1000 winstonbendariovvygn.
He based his theory on the law of conservation of mass and the law of constant composition. Law of conservation of mass is explained in Daltons atomic theory. Toluene has a density of 0866gcm3.
Are rearranged during a chemical. It means that only the distribution off atoms occurred. He said that reorganization of atoms is involved in chemical reactions.
Dalton doesnt explain the law of conservation of mass. This means mass is neither created nor destroyed in a chemical reaction ie. Daltons atomic theory was the very first complete attempt to describe all matter in terms of atoms and their properties.
Diffraction is when light is bent around obstructions. List two ways in which theories generally differ from hypothesis. 1 Get Other questions on the subject.
This law is called the Law of conservation of mass. How does Daltons atomic theory explain this law. If in a chemical.
Atoms cannot be created or destroyed. Atoms of same element can. Daltons theory was built on.
In 1808 the English physicist and chemist John Dalton proposed the atomic theory a scientific theory on the nature of matter. According to the Daltons atomic theory law of conservation of mass states that Matter is made up if extremely small indivisible particles which can neither be created nor be destroyed. No additional new atom is formed or is added except the reactions hence the mass is conserved.
Thus s theory explains the law of conservation of mass as follows. The total mass of materials present before a chemical reaction is the same as the mass of materials after. How is a theory different from a hypothesis.
Thats to start with. His law which is related to multiple proportions is part of the. Items of an element are identical in size not send the other properties items of different elements different size mass and other properties.
1 Every element is composed of minute particles called as atoms. Mass can neither be created nor destroyed. Since it states that atoms cannot be created or destroyed Daltons theory suggests that the net mass of the participating species in a chemical reaction is conserved.
Are rearranged during a chemical reaction. How does Daltons atomic theory explain the law of conservation of mass. How did Daltons Atomic Theory explain the Law of Conservation of Mass.
Convert 633 x 108µg to kg. We need to explain how the Daltons theory explained them. How does daltons atomic theory account for the law of mass conservation.
Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. State and explain the following i Atom ii Molecule iii Atomic mass iv Molecular mass 68. Arrange the following in roder of increasing masses i 01 g atom of silver ii 01 mole of H 2 SO 4 iii 10 23 molecule of CO 2 gas iv 1 gram of carbon v atoms of calcium.
Daltons atomic theory helped to explain the law of conservation of mass because it stated that atoms. The first part of the theory states that all matter is made of atoms which are indivisible. Atoms of different elements may combine with each other in a fixed simple whole number ratio to form compound atoms.
It states that matter can neither be created nor destroyed. State and explain Law of Conservation Mass. Which of the these observation about clouds would indicate diffraction.
Atoms of the same element are similar in shape and mass but differ from the atoms of other elements.

3 Fundamental Laws Of Chemistry Law Of Conservation Of Mass Dalton S Atomic Theory Teaching Chemistry Atomic Theory Conservation Of Mass

Atomic Models And Their Innovations Atomicmodel Atomicmodels Daltonmodel Thomsonmodel Rutherfordmodel Bohrmod Chemistry Lessons Atom Chemistry Classroom

Chemical Bonding The Law Of Conservation Of Mass Britannica

Dalton S Atomic Theory Article Khan Academy

Solved Is Dalton S Theory Consistent With The Law Of Conservation Of Mass Yes Because Number Of Atoms Doesn T Change In Chemical Reactions No Dalton S Theory Allows Change Of Mass Only In Physical Processes

Which Postulate Of Daltons Atomic Theory Can Explain The Law Of Definite Proportions Youtube

Chemistry 101 The Three Laws That Led To Daltons Atomic Theory Youtube
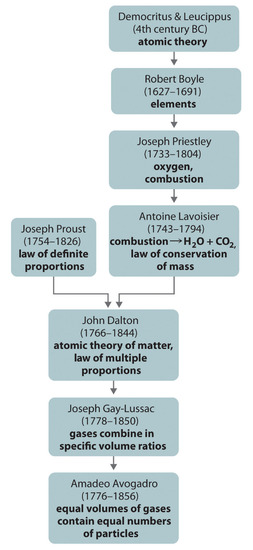
2 2 Scientific Laws Conservation Of Mass And Definite Proportions Chemistry Libretexts

Entry 4 Conservation Of Mass Chemical Reactions Atomic Theory

Which Postulate Of Daltons Atomic Theory Is The Result Of The Law Of Conservation Of Mass Youtube
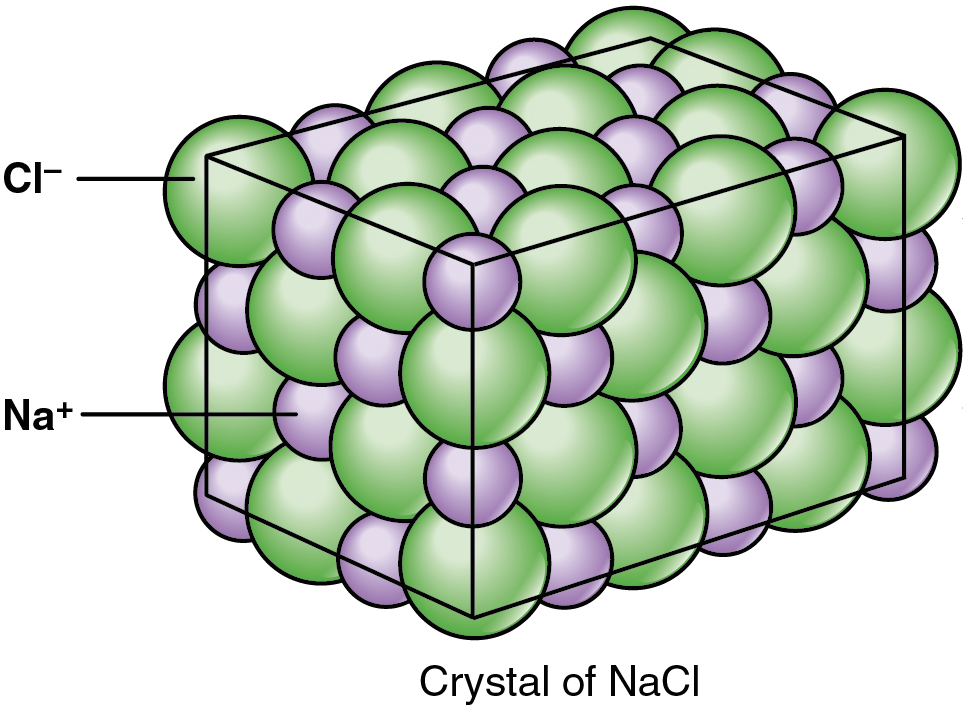
Dalton S Atomic Theory Article Khan Academy

Select A Section Introduction Atoms Molecules And Ions Laws And Theories A Brief Historical Introduction 2 1 Laws Of Chemical Combination 2 2 John Dalton And The Atomic Theory Of Matter 2 3 The Divisible Atom 2 4 Atomic Masses 2 5 The Periodic

Dalton S Atomic Theory Overview Modern Application Expii

Atomic Theory Lesson Atomic Theories Discoveries In History Atomic Theory Science Teaching Resources Atom

What Is John Dalton S Atomic Model Universe Today

Dalton S Atomic Theory Postulates Limitations Merits Chemistrygod



Comments
Post a Comment